- Hits: 2217
Cleaning/Tableting/Recycling Pilot plant
1. Cleaning process
Tank cleaning is the most essential in the industries. There is usually a buildup of contamination on the reactor walls and on the agitator blade surface, which can be easily cleaned with different methods.
- Hydro -blasting:
Hydro- blasting can be utilized to clean the insides of pipes, tanks, and process reactors.
Our process uses high pressure water jetting (15,000 psi to 50,000 psi) to remove scaling from the internal surfaces of pipe. This process is performed on steam piping prior to steam and air blowing of the steam lines to reduce the time, water, and fuel required to clean the steam piping.
The nozzle can be rotated 360° on a horizontal or vertical plane to form a crisscross pattern to thoroughly clean the tank and remove the stickiest residue
- cryogenic cleaning
Dry ice cleaning consists of projecting particles of ice or dry ice, solid CO2 at -78°C, by a flow of compressed air onto a surface to be cleaned. The combination of intense cold and mechanical shock causes the dirt to detach from its support.
- the particles of ice or dry ice are accelerated by a flow of compressed air.
- localized thermal shocks weaken the pollutants
- cracks form and the pollutant cracks with the cold
- the micro- excavators strike the surface at a speed of 300 m/s and remove the loosened pollutants
- chemical cleaning
Cleaning is achieved by physical action of high velocity flow, jet sprays, agitation and chemical action of cleaning agents enhanced by heat. While mechanical forces are necessary to remove gross soil and to ensure adequate penetration of cleaning solutions to all areas.
three steps: initial rinse, cleaning with detergent(s) and final rinse.
- Detergent: Alcon ox™ Liquinox™
Use to clean: pharmaceutical apparatus, industrial parts, pipes,tanks and reactor.
Surfaces cleaned: Corrosion inhibited formulation recommended for
glass, metal, stainless steel.
Directions: Make a fresh 1% solution (2 1/2 tbsp per gal, 1 1/4 oz per
gal, or 10 ml per L) in cold, warm or hot water. If available, use warm
water. Use cold water for blood stains. For diffi cult soils, raise water
temperature and use more detergent. Clean by soak, circulate,
wipe…RINSE THOROUGHLY—preferably with running water.
- CIP process:
- STEP 1: PRE-RINSE
The pre-rinse is a very important step in the CIP
The pre-rinse cycle:
- Wets the interior surface of the lines and tank
- Removes most of the remaining residue
- Dissolves sugars and partially melts fats
- Provides a non-chemical pressure test of the CIP flow path
Use potable plant water, de-ionized water (DI).
A Turbidity Sensor may be used to verify that the pre-rinse effectively
removes all solids.
- STEP 2: CAUSTIC WASH – (140° – 185° F)
Caustic washes soften fats, making them easier to remove. Also known as caustic soda, sodium hydroxide or NaOH.
Caustic is typically used as the main detergent in most CIP wash cycles. A non-foaming formulation can help reduce pump cavitation and increase efficiency.
Water Saving Tip: In many cases, the caustic wash can be returned to its tank and re- used multiple times, which significantly reduces water, chemical, and energy costs over a single tank system.
- STEP 3: INTERMEDIATE RINSE
Fresh water flushes out residual traces of detergent remaining from the caustic wash.
- Level Transmitters and Probes monitor tank levels of wash and rinse tanks.
- Flow Transmitters ensure optimum flow for spray devices to precisely control wash and rinse steps.
- Conductivity Transmitters ensure chemical levels are hitting predetermined set point.
- STEP 4: FINAL RINSE
Rinse with either DI, RO, or city water to flush residual cleaning agents.
Note! the final rinse water may be recovered and reused as the pre-rinse solution for the next cleaning cycle.
- STEP 5. SANITIZING RINSE
May be required to help kill microorganisms before starting the next production run. The PAA solution:
- Is a strong disinfectant even at low temperatures.
- Rinses away while leaving little or no chlorine residue to corrode stainless steel.
- Is effective against all microorganisms including spoilage organisms, pathogens and bacterial spores.
- It has also proven to be eco-friendlier in the wastewater stream.
- Has a strong, pungent odor so it should only be used in well-ventilated areas.
- Warning: Sanitizers reduce bacterial growth but don’t completely kill all pathogens in the system; since it is the last step in the cleaning process, re- circulating the sanitizing solution could run the risk of spreading any leftover contamination that might be present. Sanitizers can also be sensitive to high temperatures and can lose their effectiveness fairly rapidly once they are in solution.
Note! Peracetic acid should be safe with stainless steel
finally we decide to use hydro-blasting method by dividing this method into three steps : pre-rinse addition of detergent, and final rinse.
2. Aspirin tableting process
Tableting aspirin is beneficial because it makes the medication easier to swallow and ensures a consistent dose. It also helps to prevent the medication from breaking down too quickly, allowing it to be stored for longer periods of time.
-First method:
Procedure:
A stable aspirin tablet may be prepared under the conditions where RH ( Relative humidity is amount of water present in an air particle over can be measured by hygrometer) is maintained below 30 %, by the process comprising steps of:
- Sifting aspirin, and microcrystalline cellulose or corn starch.
- Blending the material of step 1.
- Sifting the pregelatinized (Examples of binders include pregelatinized starch: polyvinyl pyrrolidone) and Silica colloidal anhydrous (Example of lubricant and glidant) and adding to material of step 2.
- Sifting the stearic acid and adding to material of step 3 and blending.
- Compressing the blend of step 4, The above blend was compressed using approved punches and dies.
- Preparing the enteric coating dispersion by adding and mixing talc hydrated magnesium silicate (apply a safe barrier against contamination as a glidant to improve powder flow in tablet compression), meth acrylic acid-ethyl acrylate copolymer (1:1), triethylcitrate and simethicone emulsion in water
- Spraying the dispersion onto the tablet.
Formula:
|
Ingredients |
Amount (mg) |
Role |
|
Aspirin |
75.00 |
Active ingredient |
|
Corn starch |
24.20 |
Disintegrant |
|
Starch Pregelatinized |
7.70 |
Binder |
|
Silica Colloidal Anhydrous |
2.20 |
Anticaking agent, emulsion stabilizer, glidant |
|
Stearic Acid |
0.90 |
Aspirin against degradation |
|
Talc |
3.33 |
Giladant |
|
Meth acrylic Acid-Ethyl Acrylate Copolymer (1:1) |
10.50 (As dry substance) |
Tablet binder, tablet coating agent |
|
Triethyl Citrate |
1.05 |
It allows a slower release of the contents of the tablets |
|
Simethicone Emulsion |
0.12 (As dry substance) |
Emulsion |
|
Purified Water |
q.s (Quantum satis=the amount which is enough) |
used in Preparation of enteric coating
|
-Second method
Requirement:
- Chemicals: Aspirin, starch, polyvinyl pyrrolidone, ethanol, magnesium stearate, talc.
- Glass wares: measuring cylinder, beaker, mortar and pestle, granulating sieve…
- Equipment: tray dryer, tablet press.
Procedure:
1. Weight and pass aspirin and starch powder through 60#sieve (size with a 0.0098" (250μm) nominal sieve opening with a typical wire diameter of 0.16mm).

2. Mix aspirin and starch uniformly in mortar and pestle

3. Prepare 10% PVP solution in ethanol and stir until it becomes clear

4. Add PVP solution dropwise in mortar to get cohesive mass

5. Screen prepared cohesive mass through 10#granulating sieve (a medium size U.S. Standard mesh size with a 0.0787" (2mm) nominal sieve opening with a typical wire diameter of 0.9mm) and collect it on granulating tray


6. Dry granules in tray dryer at 50Co for 30min

7. Blend granules with remaining ingredients (talc and magnesium stearate) using polybag

8. Store prepared granules in well closed and labelled container
Note: Corn starch is most suited as a vehicle for tablet compression in the pharmaceutical industry.PVP improving the bioavailability and stability of drugs, improving the physic mechanical properties of preparations, adjusting the release rate of drugs.
Formula:
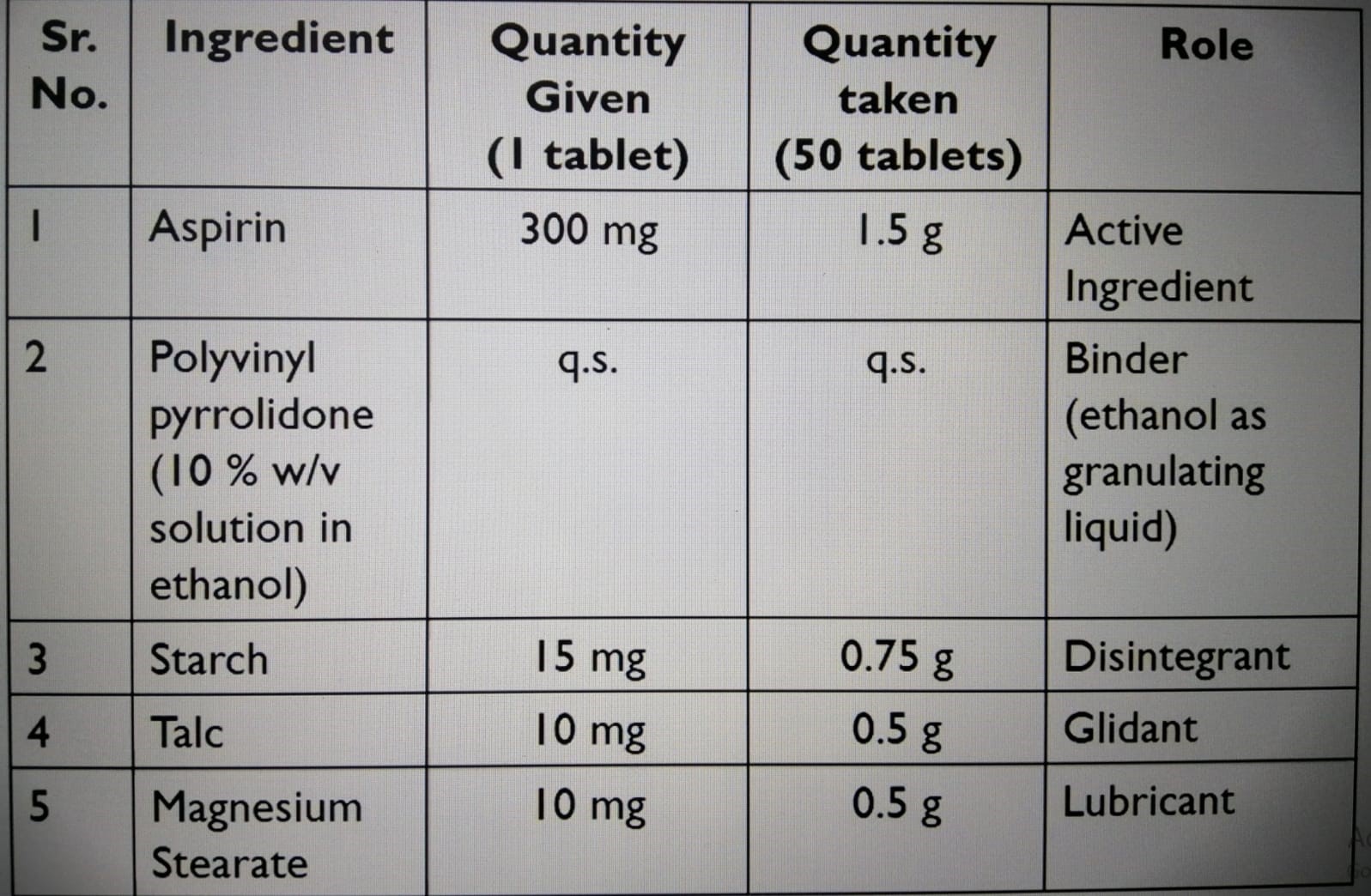
Note: this table contains a mistac in the quantity taken of aspirin (50 tablets) 15g instead of 1.5 g
To watch a video explaining click on this link :
https://www.youtube.com/watch?v=r_3bSQd2xv0&t=1453s
3. Evaluation test
A.Evaluation of granules
The Prepared Tablet is Evaluated in terms of bulk density, tapped density, the angle of repose, Carr’s Index and, hardness test, weight variation test, friability test and in vitro study. The result associated with optimized batch is good satisfactory and having better drug release kinetic.
We will have used the method and the into evaluated granules: bulk density, tapped density, the angle of repose and Hausner’s ratio.
A-A volume of powder is filled into a graduated glass cylinder and repeatedly tapped for a known duration. the volume of powder after tapping is measure.
1.Bulk density=weight /bulk volume (Bulk density: This includes the volume of solid fraction of particles and intra- and interparticulate volumes, Bulk density is defined as the mass of the many particles of the material divided by the total volume they occupy).
2.tapped density =weight / tapped volume (The tapped density is an increased bulk density attained after mechanically tapping a container containing the powder sample)
The tap density tester is ideal for measuring the tapped density of powders:

B.The Hausner’s ratio is a number that is correlated to the flow ability of a powder or granular material. The Hausner ratio is used in a wide variety of industries as an indication of the flow ability of a powder.
Hausner’s ratio=Tapped density /bulk density
A Hausner Index greater than 1.2 is considered an indication of poor flow but good compressibility and good cohesion.
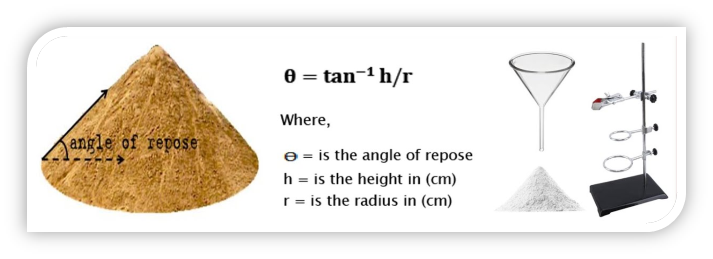
C. Angle of repose is a parameter commonly used for the evaluation of interparticle force, Angle of rest is defined as the angle that an inclined plane makes with the horizontal when a body placed on it just starts sliding.
How can be calculate angle of repose?
The simplest method for the determination of the angle of repose is the “poured” angle. A funnel with a wide outlet is affixed at a distance of 10 cm above a piece of paper is placed directly beneath the funnel. Powder is added while the funnel is closed. The contents flow through and collect on the paper. The diameter of the cone (D) and two opposite sides (l1 + l2) are measured with rulers. The angle of repose (θ) is calculated from the equation arc cos [D/(l1 + l2)]. The relationship between flow properties and angle of repose has been established. !!When the angle of repose is less than 25 degrees, the flow is said to be excellent; on the other hand, if the angle of repose is more than 40 degrees, the flow is considered to be poor.
B. Evaluation of tablets
Disintegration test for tablets:
The disintegration test is used to show how quickly the tablet breaks down into smaller particles, allowing for a greater surface area and availability of the drug when taken by a patient.
To carry out a disintegration test for tablets, we use a basket which holds 1 to 6 tablets. This is then raised and lowered into a beaker of water, which is used to simulate conditions in the stomach at 37°C. If the tablets or capsules float, perforated plastic disks are placed on the top of the tablets to keep them under the water level. The tablet disintegration time is taken when no residue is left in the mesh.
4. Recycling
The production of aspirin typically generates waste byproducts which can be harmful to the environment if not disposed of properly.Recycling of aspirin production waste can be done through various methods such as:
I .Recovery of salicylic acid: Salicylic acid can be recovered from the waste generated during aspirin production through a process called acidification and crystallization. Here are the basic steps involved in the process:
1.Acidification: The waste solution containing salicylic acid is first acidified with a strong mineral acid such as hydrochloric acid or sulfuric acid to convert the salicylic acid to its protonated form. The acidification is done by slowly adding the mineral acid to the waste solution while stirring until the pH of the solution reaches around 2. The protonation of salicylic acid is described by the following equation:
C7H6O3 + H3O+ → C7H7O3+ + H2O.where C7H6O3 represents salicylic acid and H3O+ represents the hydronium ion.
For example, if there is 1 gram of excess salicylic acid in the crude aspirin mixture, then approximately 1.5 to 2 grams of sulfuric acid would be added to react with the excess salicylic acid.
It's important to note that the exact quantities used can vary depending on the specific process and the conditions under which the acidification is carried out. Additionally, the acidification step must be carefully controlled to avoid the formation of undesirable byproducts or the degradation of the salicylic acid.
2.Extraction: The protonated salicylic acid is then extracted from the waste solution using an organic solvent such as ethyl acetate, dichloromethane, or toluene. The organic solvent is added to the acidified solution and the mixture is shaken or stirred for a few minutes to allow for the extraction of the protonated salicylic acid into the organic layer. The organic layer is then separated from the aqueous layer using a separating funnel. The quantities used in the extraction process (such as the amount of organic solvent or washing solution used) can vary depending on the specific process and the amount of crude aspirin being processed.
3.Recrystallization: The organic layer containing the protonated salicylic acid is then evaporated to dryness to obtain the crude salicylic acid. The crude salicylic acid is then dissolved in a suitable solvent such as ethanol or methanol and heated to dissolve the salicylic acid completely. The solution is then allowed to cool slowly to room temperature, causing the salicylic acid to recrystallize from the solution. The crystals are then filtered, washed with cold solvent to remove any impurities, and dried to obtain pure salicylic acid. the general amount used is about 3-5 times the mass of the salicylic acid being recrystallized. For example, if you have 1 gram of salicylic acid, you would use approximately 3-5 mL of ethanol as the solvent for the recrystallization step.
Once the salicylic acid has been recovered and purified, it can be reused in the production of aspirin or other products that require salicylic acid as a starting material.
II. Recycling of acetic acid:
- Reuse of acetic acid: The acetic acid produced during aspirin production can be purified and reused in subsequent batches of aspirin production. This reduces the amount of waste generated and saves on production costs,
Acetic acid is commonly used in the synthesis of aspirin as a catalyst to help facilitate the reaction between salicylic acid and acetic anhydride.
Extraction of acetic acid: in addition, acetic acid can be recovered by distillation or by extraction with an appropriate solvent such as ether or dichloromethane Once recovered and purified, acetic acid can be reused in other chemical reactions, thus reducing costs and minimizing waste from aspirin production.
Add the solvent: A small amount of solvent is added to the mixture of water and acetic acid. Mix thoroughly: The mixture is mixed thoroughly to ensure that the solvent and water/acetic acid mixture are fully in contact with each other. Allow to separate: The mixture is then allowed to separate into two layers - the aqueous layer (containing water and acetic acid) and the organic layer (containing the solvent).
Collect the organic layer: The organic layer is carefully separated from the aqueous layer and collected in a separate container.
Repeat the extraction: The extraction process may be repeated multiple times to increase the yield of acetic acid.
Recover the acetic acid: The solvent is then evaporated from the organic layer to recover the acetic acid.
- Neutralization: The waste acetic acid can be neutralized using an appropriate base such as sodium hydroxide or calcium hydroxide. This results in the formation of a salt that can be disposed of safely. the neutralization reaction of acetic acid, which is a weak acid, with a strong base, such as sodium hydroxide (NaOH), can be represented by the following chemical equation:
CH3COOH + NaOH → CH3COONa + H2O
To determine the exact amounts needed for a given neutralization reaction, one must know the concentrations of the reactants and use stoichiometry calculations to determine the amounts needed. For example: if one wishes to neutralize 10 mL of a 0.1 M acetic acid solution with 0.2 M sodium hydroxide, one would need to add 20 mL of the NaOH solution to the acetic acid while gently shaking until neutralization is complete.
III. Recycling of acetic anhydride
In the case of extracting acetic anhydride from the waste products of aspirin production, a suitable solvent is needed that will selectively dissolve the acetic anhydride. One possible solvent for this purpose is dichloromethane (also known as methylene chloride). Here are the steps for extracting acetic anhydride from the waste:
-Add the waste mixture to a separator funnel.
-Add enough dichloromethane to the separator funnel so that the mixture is fully covered.
-Close the funnel and shake it gently to allow the solvent to mix with the waste mixture.
-Wait for the mixture to settle, with the denser layer at the bottom and the lighter layer (containing the acetic anhydride) on top.
-Slowly drain off the lighter layer (the dichloromethane layer containing the acetic anhydride) into a clean container.
-Discard the heavier layer (the aqueous layer containing the waste products).
The extracted acetic anhydride can then be further purified using methods such as distillation or recrystallization.
Recrystallization of acetic anhydride can be done by following the steps:
-Dissolve acetic anhydride in an appropriate solvent such as ethanol or acetone. The amount of solvent used will depend on the amount of acetic anhydride to be recrystallized.
-Heat the solution gently until all the acetic anhydride is dissolved.
-Slowly add the recrystallization solvent, usually water or acetone, to the hot solution, stirring constantly, until the solution is saturated and crystals begin to form.
-Allow the solution to cool slowly, preferably to room temperature, to allow crystals to form.
-Filter the crystals formed using a Büchner funnel and wash with the recrystallization solvent.
-Dry the crystals in the open air or in a low temperature oven
It is important to note that the recrystallization can be carried out several times to improve the purity of the crystals.
IV. Recycling of water: Water is an important component in the production of aspirin, and it is often used in large quantities. The water used can be treated and recycled for use in subsequent batches of aspirin production. evaporation and condensation: This process involves evaporating the waste water to remove impurities and then condensing the water vapor to produce purified water. The purified water can then be reused in the manufacturing process.
note :
Extraction of salicylic acid: It is generally extracted first because it is the main compound in the reaction mixture of the synthesis of aspirin.
Extraction of acetic acid: If you need to recover the acetic acid, you can do it after extracting the salicylic acid because it is present in lesser quantity in the reaction mixture.
Extraction of acetic anhydride: If you want to recover acetic anhydride from waste from aspirin production, this can be done after recovering acetic acid, since acetic anhydride is synthesized from acetic acid. However, please note that recovering acetic anhydride from waste can be more complicated than the first two extractions.
