- Hits: 1827
Ampicillin Production and Quantification 2023
Ampicillin Synthesis Steps
Preparation of the solution of ampicillin synthesis from our crude produced penicillin:
- Measure a volume of 7.5 mL from our produced penicillin(reserved in PB solution), using a graduated cylinder
- Add 0.8g of penicillin acylase (PGA)
- Agitating for 1h
- Add 7.5ml of the ester D-(-)-a-Phenylglycine methyl ester hydrochloride, D-PGMEH (0.24g ester in 10 ml potassium phosphate buffer) to the mix
- Add 0.24g enzyme (PGA) again
- Adjust the PH for about 6.4-7 by adding NaOH
- Put the beaker containing the bar magnet on the magnetic stirrer for 22.5 hours
(Take 1ml of the filtrate for the bacterial sensibility test later A1)

Ampicillin purification and harvesting:
- Add few drops of H2SO4 to stop the enzymatic reaction and adjust the PH to 2
- Filtrate the mix using a funnel fitted with filter paper
- Add butyl acetate solvent (1Vsolvant/2Vfiltrate)(4ml/8ml)
- Let rest for 2 minutes, then the organic phase is removed and the aqueous phase is discarded after decantation
(Take 1ml from aqueous phase A2 and organic phase A3 to test the presence of ampicillin later)
- Add 2% phosphate buffer (V/V)(4ml/4ml) to the organic solution
(we obtain an aqueous phase B1 and an organic phase B2)
- Adjust the PH to 7.5 by adding NaOH



Crystallization:
- Add 2% (W/V)(~0.1g/4.3ml) NaHCO3 to the aqueous phase medium
- Cool them at 4oC for about 7 days
- Filtrate them to harvest ampicillin sodium salt
Ampicillin Quantification (Standard Protocol):
- The one bacterial strain which can be used: E. coli
- Regeneration of Escherichia coli Bacteria
Place the isolated colony of E. coli on a new standard agar petri dish and then spread it
Incubate the bacteria for approximately 18 hours in an incubator at 37°C
- Qualitative antibiogram, using disc diffusion method
To test the effectiveness of the antibiotic on the bacteria
- Take 3 to 5 colonies of the isolated colonies of E. coli with a loop
- Add them in 2ml sterile saline (NaCl 0.9%)
- Vortex the saline tube to create a smooth suspension
- Adjust the turbidity of this suspension to a 0.5 McFarland standard by adding more organism if the suspension is too light or diluting with sterile saline if the suspension is too heavy.
- Inoculate the surface of Mueller Hinton agar plate by streaking the swab 3 times over the entire agar surface
- Sit the plate at room temperature at least 3 to 5 minutes (but no more than 15 minutes) to let the surface of the agar plate dry before proceeding to the next step
- Reverse the plate and divide it into 6
- Deposit 5 disc in each quadrant with an empty quadrant used as control negative
- Add 20µl of each simple on a disc
- Reverse the plate and incubate it at at 37o for 18 to 24 h
Results and Discussion
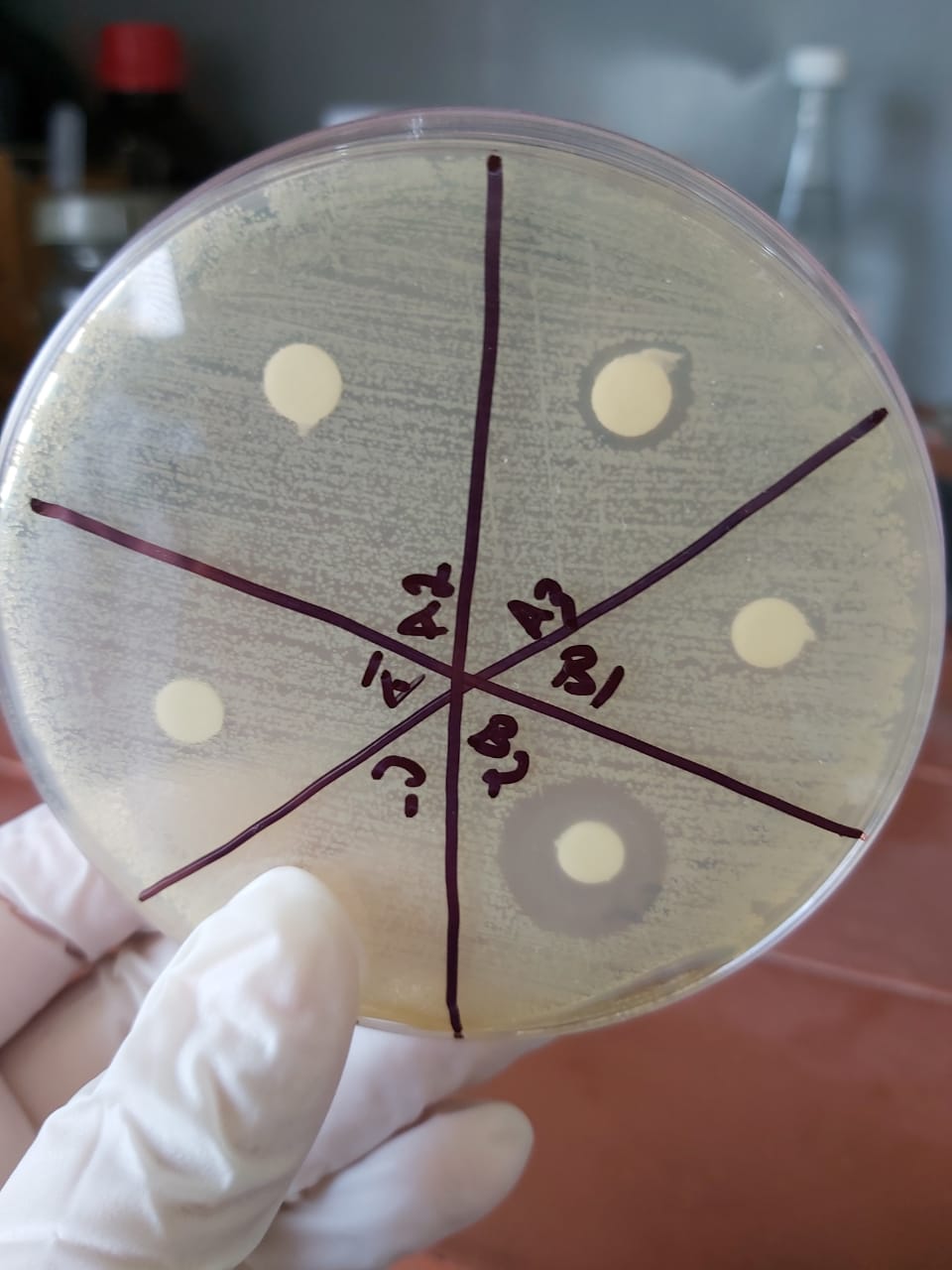
The sensitivity test shows an important zone of inhibition in A3 and B2 proving the presence of crude produced ampicillin obtained from our crude penicillin so the reaction has succeeded.
The test was validated while repeated twice.
Samples was taken from:
A1: solution obtained after the first filtration before adding the organic solvent
A2: Aqueous phase after butyl acetate extraction
A3: Organic phase after butyl acetate extraction
B1: Aqueous phase after PB extraction
B2:Organic phase after PB extraction
The results show :
- no zone of inhibition in A1(sample taken before adjusting the 2)
- No zone of inhibition in the aqueous phaseA2 so the total produced ampicillin is extracted into the organic phaseA3
- A small zone of inhibition in A3 so there is a loss while extracting ampicillin from this phase
- No zone of inhibition in the Aqueous phaseB1 with an important one in the organic phaseB2 so PB isn't the right solvent for ampicillin extraction for crystallization.
Crude Ampicillin quantification
Preparation for commercial ampicillin quantification
While the commercial ampicillin used in quantification had no results on E.coli
We decided to use amoxicillin instead since it has an identical antibacterial activity with ampicillin.

- Weigh 1g of commercial amoxicillin
- Add 5 ml of potassium phosphate buffer to the amoxicillin
- Filtrate the mix using a sterile funnel and filter paper
- Store the mix (C standard amoxicillin=200 mg/ml) in the fridge for later use
- Prepare the sterile tubes with different concentration 20, 15, 12, 10, 8 et 5 mg/mL
|
TUBES |
Solution ml |
Distilled water ml |
|
Tube1 20mg/ml |
1 |
9 |
|
Tube2 15mg/ml |
7.5 |
2.5 |
|
Tube3 12mg/ml |
6 |
1.5 |
|
Tube4 10mg/ml |
5 |
1 |
|
Tube5 8mg/ml |
4 |
1 |
|
Tube6 5mg/ml |
2.5 |
1.5 |
Quantification of the produced ampicillin using the disc diffusion method:
- Take 3 to 5 colonies of the isolated colonies with a loop, and we added them in 2ml sterile saline (NaCl 0.9%)
- Vortex the saline tube to create a smooth suspension.
- Adjust the turbidity of this suspension to a 0.5 McFarland standard(annex)
- by adding more organism if the suspension is too light or diluting with
- sterile saline if the suspension is too heavy.
- Use this suspension within 15 minutes of preparation.
- Inoculate the surface of 3 Mueller Hinton agar plate by streaking the swab 3 times over the entire agar surface, we rotated the plate approximately 60֯ each time to ensure an even distribution of the inoculum (use a control plate with E. coli on mueller Hinton agar)
- Leave the plates at room temperature at least 3 to 5 minutes (but no more than 15 minutes) for the surface of the agar plate to dry before proceeding to the next step.
- Reverse the plates and divide it into 4
- Deposit a disc in each quadrant
- Add 20µl of each concentration on a disc with the unknown one (A3 and B2)
- Reverse the plate and incubate it at at 37o for 18 to 24 h
- After the growth time, measure the zone of inhibition that had appeared using a ruler.
- Draw a graph showing the concentration of amoxicillin as a function of the diameter in order to be able to quantify the produced ampicillin (diameter as a function of Log C).
Results
Table 1 : Measurements of diameters of bacterial growth inhibition zones for different concentrations of standard dilute commercial amoxicillin and A3,B2.
|
Diameter(cm) |
6 |
2.7 |
3.5 |
3.2 |
3.4 |
3.1 |
1(A3) |
1.1(B2) |
|
LogC |
1.30 |
1.17 |
1.07 |
1 |
0.90 |
0.69 |
? |
? |
|
Concentration(mg/ml) |
20 |
15 |
12 |
10 |
8 |
5 |
? |
? |

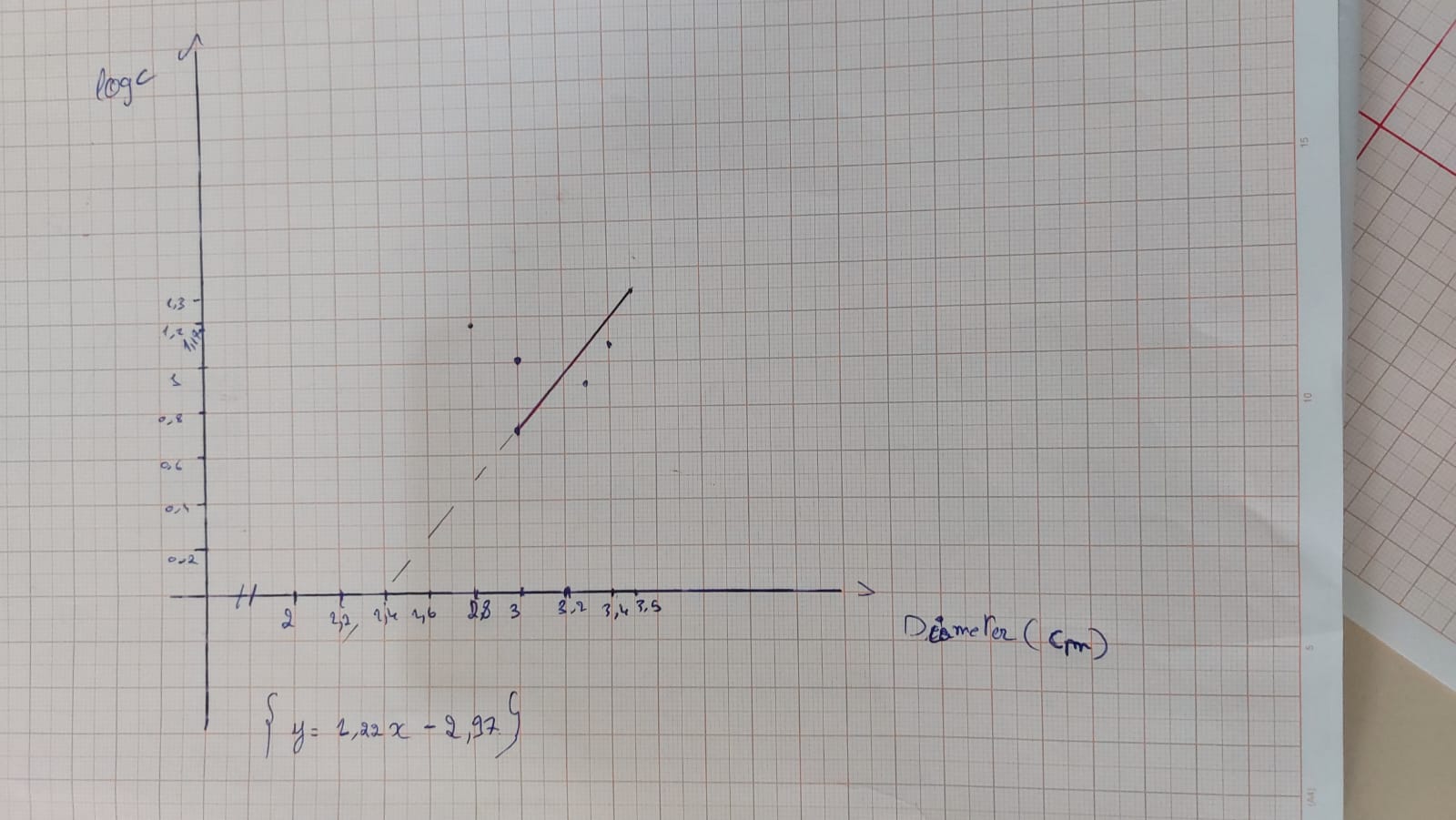
Graph 1 : Graph showing the variation of amoxicillin concentration (LogC) as function of the diameter of the inhibition zone.
According to the calibration curve y=1.22x-2.97
So for an inhibition zone of 1cm the concentration is 0.01 mg/ml
For an inhibition zone of 1.1 cm the concentration is 0.02 mg/ml
Results :
Trial 2 (6/6/2023)
Table 2: Measurements of diameters of bacterial growth inhibition zones for different concentrations of standard dilute commercial amoxicillin and A3,B2.
|
Diameter(cm) |
2.1 |
2 |
1.9 |
1.75 |
1.8 |
1.55 |
1(A3) |
0.85(B2) |
|
LogC |
1.30 |
1.17 |
1.07 |
1 |
0.90 |
0.69 |
? |
? |
|
Concentration(mg/ml) |
20 |
15 |
12 |
10 |
8 |
5 |
? |
? |

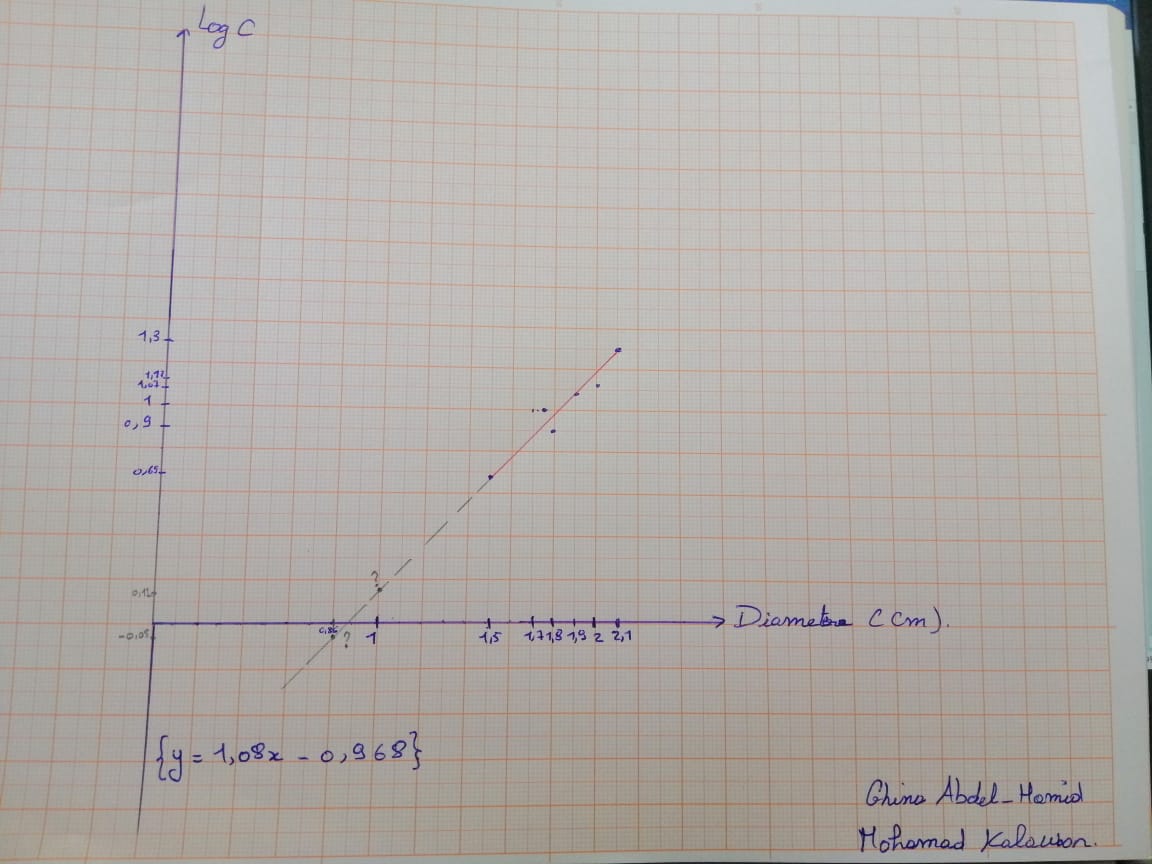
Graph 2 : Graph showing the variation of amoxicillin concentration (LogC) as function of the diameter of the inhibition zone.
According to the calibration curve y=1.08x-0.968
So for an inhibition zone of 1cm the concentration is 1.31 mg/ml
For an inhibition zone of 0.85 cm the concentration is 0.89 mg/ml
Note: -We use the commercial amoxicillin instead ampicillin
-We filtrated the commercial amoxicillin
-Uncompleted inhibition in B2
-We should do a double number of Petri dish in target to reduce the error.
